The following is an Arrhenius plot of a first-order reaction.The rate constant is measured in units of s-1. 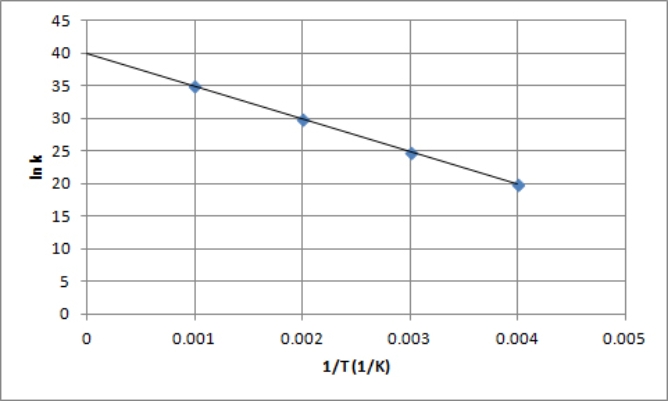 Based on this Arrhenius plot,what is the Arrhenius frequency factor (A) of the reaction? (R =8.314 J/K mol)
Based on this Arrhenius plot,what is the Arrhenius frequency factor (A) of the reaction? (R =8.314 J/K mol)
Definitions:
Statistically Significant
A term indicating that the results of an analysis are unlikely to be due to chance, according to a predefined threshold of probability.
Atheoretical
Lacking a theoretical basis, not guided by a theory.
Empirical
Based on observations or experience rather than theory or pure logic.
Rationally Constructed
Developed or created based on logical reasoning and thoughtful consideration.
Q7: Children become overweight or obese because they
Q11: Mental health<br>A)allows children to effectively cope with
Q14: Risk factors for dental caries does not
Q16: _ are atoms that have the same
Q17: Two isotopes of an element differ only
Q27: Some ethnic groups suffer disproportionately more from
Q42: The units of the rate constant depend
Q51: What terms defines a mass which is
Q109: _ is the name given for the
Q137: A(n)_ is a substance that has a