Multiple Choice
In the following Lewis structure for ClO3F, chlorine has a formal charge of ____ and an oxidation number of ____. 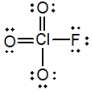
Definitions:
Related Questions
Q8: Predict the products for the following set
Q9: Hydrogen fluoride is used<br>A) in the manufacture
Q11: A molecule that contains polar bonds will
Q38: A trade show is a way for
Q40: Compare the traditional one-to-many marketing communication model
Q67: All possible resonance structures contribute equally to
Q77: Which advertising agency specialist is responsible for
Q84: Select the correct name for the following
Q116: Which of the following would be the
Q144: Which of the following is a service