Calculate the standard cell potential ( 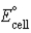 ) for the reaction 2 Ag(s) + Co2+(aq) → 2 Ag+(aq) + Co(s) . The standard reduction potentials are as follows:
) for the reaction 2 Ag(s) + Co2+(aq) → 2 Ag+(aq) + Co(s) . The standard reduction potentials are as follows:
Ag+(aq) + e−→ Ag(s)
E° = 0.8 V
Co2+(aq) +2 e−→ Co(s)
E° = -0.277 V
Definitions:
Three-point Gait
A method of walking or moving with the support of crutches or a walker, where one leg and both supports touch the ground simultaneously.
Axillae
The underarm area of the human body; plural form of axilla.
Crutch Handgrips
The part of a crutch that is held by the hand, designed to provide support and stability for individuals with mobility issues.
Myoneural Junction
The point of contact between a nerve and the muscle it controls, facilitating the transmission of signals that cause muscle contraction.
Q7: What is the formula for dicyanobis(ethylenediamine)zirconium(IV)nitrate? (en
Q18: For which of the following reactions will
Q30: Nitrogen trifluoride decomposes at to form nitrogen
Q44: What is the freezing point of a
Q52: The rate constant at 366 K for
Q63: Which of the following is the correct
Q66: Carbonic acid is a diprotic acid,H<sub>2</sub>CO<sub>3</sub>,with K<sub>a1</sub>
Q78: Which list contains the three most abundant
Q85: The N atom in an amide has
Q162: The number of protons in the nucleus