At 25 °C,the decomposition of dinitrogen tetraoxide N2O4(g) 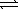 2 NO2(g)
2 NO2(g)
Has an equilibrium constant (Kp) of 0.144.At equilibrium,the total pressure of the system is 0.0758 atm.What is the partial pressure of each gas?
Definitions:
Heptane
A straight-chain alkane with seven carbon atoms in its molecule, often used as a nonpolar solvent in laboratories.
Moles
A unit of measurement used in chemistry to express amounts of a chemical substance, defined as containing exactly 6.02214076×10^23 particles.
Newman Projection
A method of visualizing the conformation of a chemical compound by projecting its bond structure along a chosen bond axis.
Anti Conformation
A spatial arrangement in molecules where substituents on adjacent carbons are positioned as far apart as possible, reducing steric strain and often seen in Newman projections.
Q12: Which of the following statements about entropy
Q16: A possible mechanism for the gas phase
Q33: Which of the following is the correct
Q42: A solution containing 10.mmol of CO<sub>3</sub><sup>2</sup><sup>−</sup>and 5.0
Q47: Nitric acid,HNO<sub>3</sub>,dissociates in water to form nitrate
Q47: Claculate the mass of chromium that can
Q57: What is the mole fraction of calcium
Q60: What type of colloid is formed when
Q70: The hydroxide ion concentration of a saturated
Q83: Which of the following is the balanced