The standard free energy change associated with the dissolution of ammonium nitrate in water is -6.73 kJ/mol at 298.15 K. NH4NO3(s) 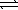 NH4NO3(aq)
NH4NO3(aq)
Which of the following is the equilibrium constant for the reaction? (R = 8.314 J/K⋅mol)
Definitions:
Minimum Wage
The lowest legally permissible amount that workers can be paid for their labor, designated by government law or regulation.
Part-Time Work
Employment characterized by working fewer hours than those considered full-time, often offering greater flexibility but less job security and benefits.
Intimate Partner Violence
Violence or abuse by one person against their partner in an intimate relationship, encompassing physical, sexual, emotional, and psychological abuse.
Immigrant Women
Female migrants who have moved from their country of origin to another country, facing unique challenges, opportunities, and contributions within their new communities.
Q6: The cell potential of the following electrochemical
Q6: The side chains of nonpolar amino acids
Q7: The reaction quotient,Q,for a system is <img
Q7: Calculate the enthalpy of vaporization of water
Q12: A molecule will always be polar if
Q23: When 0.20 mole HF is dissolved in
Q41: What is the balanced chemical equation for
Q41: Which of the given relationships correctly compares
Q45: Using the given data,determine ΔrG° at 500.0
Q72: How many moles of solid NaF would