When 0.20 mole HF is dissolved in water to a volume of 1.00 L,5.8% of the HF dissociates to form F-(aq) .What is the equilibrium constant for the reaction? HF(aq) + H2O( 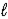 )
) 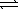 F-(aq) + H3O+(aq)
F-(aq) + H3O+(aq)
Definitions:
Liberal-Left Agenda
A term often used to describe political policies and philosophies that advocate for social equality, environmental protection, and governmental intervention in the economy to support welfare programs.
United Nations
An intergovernmental organization established in 1945 to promote international cooperation and to achieve peace and security.
Wartime Manufacturing
The production of military equipment and supplies during periods of war.
American Business
Economic activities and enterprises based in the United States, contributing to its GDP and employment.
Q9: What is the formula of the common
Q12: In which of the following reactions does
Q20: What mass of Na<sub>2</sub>SO<sub>4</sub> must be dissolved
Q29: Which of the following is not a
Q34: For any process,the change in entropy of
Q35: The rate constant for a reaction at
Q47: Which of the following expressions correctly describes
Q51: In HF<sub>2</sub>− the hydrogen is shared between
Q53: Given the following chemical equilibria, N<sub>2</sub>(g)+ O<sub>2</sub>(g)
Q76: Write a balanced chemical equation for the