For the reaction given below,ΔH0 = −1516 kJ at 25°C and ΔS0 = −432.8 J/K at 25°C.This reaction is spontaneous _____. SiH4(g) + 2 O2(g) → SiO2(s) + 2 H2O 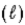
Definitions:
Organization Chart
A visual representation of the hierarchical structure of an organization, detailing relationships among its parts or positions.
Closed-System Control
An approach to management or control that is self-contained and does not interact with external environments, focusing on internal functioning.
Diagonal Communication
Information exchange that occurs between individuals at different levels and different departments within an organization, bypassing traditional hierarchical structures.
Vertical Control
A management approach where decision-making and directives flow from the top levels of the organizational hierarchy down to the lower levels.
Q21: Biodiesel is a mixture of:<br>A) fats and
Q35: The approximate rise in the surface temperature
Q43: At 800 K,the equilibrium constant,K<sub>p</sub>,for the following
Q47: Which of the following will not as
Q47: The rate law for a reaction is
Q53: Given the following chemical equilibria, N<sub>2</sub>(g)+ O<sub>2</sub>(g)
Q58: Brønsted-Lowry acid-base reactions are restricted to only
Q78: The chemical formula of _ is K<sub>2</sub>[PtCl<sub>4</sub>].<br>A)
Q83: What is the concentration of silver(I)ion in
Q100: What is the most abundant metal on