Calculate the molar concentration of uncomplexed Zn2+(aq) in a solution that contains 0.17 M Zn(NH3) 42+ and 0.3775 M NH3 at equilibrium.Kf for Zn(NH3) 42+ is 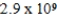 .
.
Definitions:
Slash-And-Burn Horticulture
A farming method that involves cutting and burning plant life to create fields for agriculture.
Equivalent Value
A concept referring to the equality of value between two entities, often used in economics and exchange theories.
Hard Bargaining
A negotiation strategy involving firm stands on demands and little willingness to compromise.
Reciprocity
A principle of mutual exchange in social relations where benefits or services are returned in kind.
Q6: If the ratio of acid to base
Q7: What is the molar solubility of Mn(OH)<sub>2</sub>(s)in
Q15: In any chemical process,energy must be conserved.This
Q20: What is the highest oxidation state possible
Q33: The complex ion [NiCl<sub>4</sub>]<sup>2-</sup> is paramagnetic,but the
Q50: The majority of electricity generated in the
Q53: Given the following chemical equilibria, N<sub>2</sub>(g)+ O<sub>2</sub>(g)
Q65: Consider the following equilibrium: CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img
Q68: The standard enthalpy of formation of ammonia
Q70: Write the expression for K<sub>p</sub> for the