Given that Ka for the weak acid HA is 3.49 × 10-8,calculate K for the reaction of HA with OH-. HA(aq) + OH−(aq) D A−(aq) + H2O( 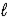 )
)
Definitions:
Adjusting Entry
An accounting entry made at the end of an accounting period to allocate income and expenditure to the correct period.
Prepayment Accounts
Accounts used to record expenses that have been paid in advance and are recognized incrementally as expenses over time.
Balance Sheet
A financial statement that displays a company's financial position at a specific point in time, including assets, liabilities, and shareholders' equity.
Income Statement
A financial statement that shows a company's revenues and expenses over a specific period, leading to its net income or loss.
Q5: A 2.5 L flask is filled with
Q8: Termolecular elementary steps are rare.Why?
Q9: A sample of helium gas occupies 17.9
Q9: Which of the following is the correct
Q10: The osmotic pressure of blood is 7.65
Q20: Which of the following liquids has the
Q22: Carbonic acid is a diprotic weak acid.Which
Q27: Hydrogen gas is prepared by electrolysis of
Q30: How many sigma (σ)bonds and pi (π)bonds
Q64: At 25 °C,0.138 mg AgBr dissolves in