Given the following chemical equilibria, N2(g) + O2(g) 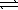 2 NO(g)
2 NO(g)
K1
N2(g) + 3 H2(g) 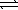 2 NH3(g)
2 NH3(g)
K2
H2(g) + 1/2 O2(g) 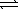 H2O(g)
H2O(g)
K3
Determine the method used to calculate the equilibrium constant for the reaction below.
4 NH3(g) + 5 O2(g) 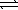 4 NO(g) + 6 H2O(g)
4 NO(g) + 6 H2O(g)
K c
Definitions:
Lockbox System
A service provided by banks to companies for the receipt of payment from customers, where payments are sent to a special post office box rather than to the company.
Treasury Bills
Short-term government securities issued at a discount from their face value, with maturities ranging from a few days to 52 weeks.
Collection Time
The average duration it takes for a company to collect payments owed by its customers, often measured in days.
Average Daily Receipts
The average amount of money a business receives on a day-to-day basis over a specified period.
Q11: A gas sample is heated from −20.0°C
Q12: What is the molar solubility of solid
Q13: Calculate E<sub>cell</sub> for the following electrochemical cell
Q24: The electron configuration of a particular diatomic
Q27: Which of the following reactions will require
Q27: The decomposition of formic acid follows first-order
Q39: Which of the following releases the largest
Q57: For the formation of 1 mol of
Q57: In the context of valence shell electron
Q62: If Δ<sub>r</sub>G° for the following reaction is