A 3.50-mol sample of HI is placed in a 1.00-L vessel at 460°C,and the reaction system is allowed to come to equilibrium.The HI partially decomposes,forming 0.266 mol H2 and 0.266 mol I2 at equilibrium.What is the equilibrium constant Kc for the following reaction at 460°C? ½ H2(g) + ½ I2(g) 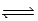 HI(g)
HI(g)
Definitions:
External Rewards
Reinforcements or incentives from the external environment that motivate behavior or actions.
Regulate
The act of controlling or maintaining the rate or speed of a process or system, often to achieve stability or to meet certain criteria.
Adjusting
Adjusting entails modifying or altering behaviors, strategies, or attitudes to better fit new conditions or to achieve a desired outcome.
Kindergarten
A preschool educational approach that emphasizes play, physical activity, creativity, and socialization to foster children's growth and development.
Q2: Sodium chloride crystallizes in a(n)_ cubic unit
Q17: What is responsible for capillary action,a property
Q24: A 3.50-mol sample of HI is placed
Q32: What mass of ethylene glycol,when mixed with
Q40: What concentration of silver nitrate (in ppm)is
Q46: Desalination of seawater by reverse osmosis requires
Q64: The best explanation for the existence of
Q71: When gaseous carbon monoxide and hydrogen are
Q77: Which of the following equations illustrates the
Q79: Ammonium perchlorate can decompose violently according to