An aqueous mixture of phenol and ammonia has initial concentrations of 0.200 M C6H5OH(aq) and 0.120 M NH3(aq) .At equilibrium,the C6H5O-(aq) concentration is 0.050 M.Calculate the equilibrium constant,K,for the reaction below. C6H5OH(aq) + NH3(aq) 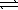 C6H5O- + NH4+(aq)
C6H5O- + NH4+(aq)
Definitions:
Direct Labor Variances
The difference between the expected (standard) cost of direct labor for actual production and the actual cost incurred.
Direct Labor
Direct labor involves the work of employees who are directly involved in producing goods or services, being a key factor in manufacturing costs.
Labor Efficiency Variance
The discrepancy in the actual worked hours versus the expected standard hours, multiplied by the standard rate for labor.
Direct Labor
The labor costs directly associated with the manufacture of specific goods or the provision of services, typically consisting of wages paid to production workers or technicians.
Q6: Which of the following molecules or ions
Q14: What is the freezing point of a
Q17: What is responsible for capillary action,a property
Q28: Molecules must overcome a barrier called the
Q43: Elemental sodium is commercially produced by electrolysis
Q51: A surfactant used for cleaning is called
Q52: The rate constant at 366 K for
Q64: Calculate the equilibrium constant for the reaction
Q77: Which of the following has a Lewis
Q89: A glass column is filled with mercury