Exactly 1.0 mol N2O4 is placed in an empty 1.0-L container and allowed to reach equilibrium described by the equation N2O4(g) 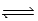 2NO2(g) . If at equilibrium the N2O4 is 27.0% dissociated,what is the value of the equilibrium constant,Kc,for the reaction under these conditions?
2NO2(g) . If at equilibrium the N2O4 is 27.0% dissociated,what is the value of the equilibrium constant,Kc,for the reaction under these conditions?
Definitions:
1950s
The decade spanning from January 1, 1950, to December 31, 1959, marked by significant events in politics, society, culture, and technology during the Cold War era.
Suburban America
Refers to the residential areas surrounding urban centers, characterized by lower population density, single-family homes, and often a higher standard of living.
Termination Policy
A set of laws or regulations designed to assimilate Native Americans into mainstream American society, often by ending the recognition of Native American tribes and their sovereign rights.
Eisenhower Administration
The presidency of Dwight D. Eisenhower, the 34th President of the United States, from 1953 to 1961, noted for its focus on moderate domestic policies and a strong stance against communism.
Q8: How many sigma and pi bonds are
Q13: Which of the following nitrogen oxides are
Q21: Write a balanced half-reaction for the reduction
Q23: The space-filling representation provided below is an
Q35: At a high temperature,equal concentrations of 0.160
Q48: What is the total number of electrons
Q54: Gold and platinum are commonly used as
Q59: Which of the following is the third
Q60: Which of the following substances is never
Q62: What molar ratio of acetic acid to