Determine the enthalpy change for the decomposition of calcium carbonate CaCO3(s) → CaO(s) + CO2(g)
Given the thermochemical equations below.
Ca(OH) 2(s) → CaO(s) + H2O( 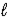 )
)
ΔrH° = 65.2 kJ/mol-rxn
Ca(OH) 2(s) + CO2(g) → CaCO3(s) + H2O( 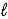 )
)
ΔrH° = −113.8 kJ/mol-rxn
C(s) + O2(g) → CO2(g)
ΔrH° = −393.5 kJ/mol-rxn
2 Ca(s) + O2(g) → 2 CaO(s)
ΔrH° = −1270.2 kJ/mol-rxn
Definitions:
Gross Profit
The difference between sales revenue and the cost of goods sold, before deducting overhead, payroll, taxes, and interest expenses.
Beginning Inventory
The worth of merchandise ready for purchase at the beginning of a financial period, prior to any acquisitions being made or deductions for sales.
Ending Inventory
The concluding amount of products ready for purchase at the close of a financial cycle.
LIFO Cost Flow
A method used in inventory management and accounting where the last items placed in inventory are the first ones to be recorded as sold.
Q1: The absolute zero point on the Kelvin
Q11: An ionic compound has the formula MCl<sub>2</sub>.The
Q17: What is the bond angle in a
Q22: Potassium hydrogen carbonate decomposes according to the
Q25: Which of the following atoms is diamagnetic
Q44: If 0.1800 g of impure soda ash
Q66: Which of the following elements is not
Q67: Which species is reduced in the reaction
Q71: Pure copper may be produced by the
Q79: What is the molecular geometry around the