9.26 g of an unknown solid is dissolved in a coffee cup calorimeter that contains 62.0 mL of water. The measured temperature increased from 23.4 °C to 24.7 °C. The specific heat of water is 4.184 J g-1 °C-1, and assume the density of the solution is 1.00 g mL-1. Calculate the molecular weight of the unknown solid if the 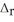 H was determined to be -2.51 kJ mol-1 via another experiment.
H was determined to be -2.51 kJ mol-1 via another experiment.
Definitions:
Detoxification
The process of removing toxic substances or qualities, often used in the context of alcohol or drug misuse, as the body metabolizes and eliminates harmful substances.
Withdrawal
The process of discontinuing a drug or substance to which one has become dependent, often leading to a range of physical and psychological symptoms.
Melatonin
A hormone produced by the pineal gland that regulates sleep patterns by signaling the body to prepare for sleep as the environment darkens.
Hormone
Hormones are chemical messengers produced by glands in the endocrine system, traveling through the bloodstream to tissues or organs, affecting many different processes, including growth, metabolism, and mood.
Q10: Use the standard reaction enthalpies given below
Q24: Determine the density of O<sub>3</sub> gas at
Q61: Identify the ground-state electron configuration for Mg<sup>2</sup>⁺.<br>A)
Q83: Consider the following balanced reaction. What mass
Q91: How many valence electrons does an atom
Q97: Use the standard reaction enthalpies given below
Q103: Place the following types of electromagnetic radiation
Q149: Charles's law<br>A)PV = nRT<br>B)V<sub>1</sub>/T<sub>1</sub> = V<sub>2</sub>/T<sub>2</sub><br>C)V<sub>1</sub>/n<sub>1</sub> =
Q192: Determine the molarity of a solution formed
Q218: What precipitate is most likely formed from