Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 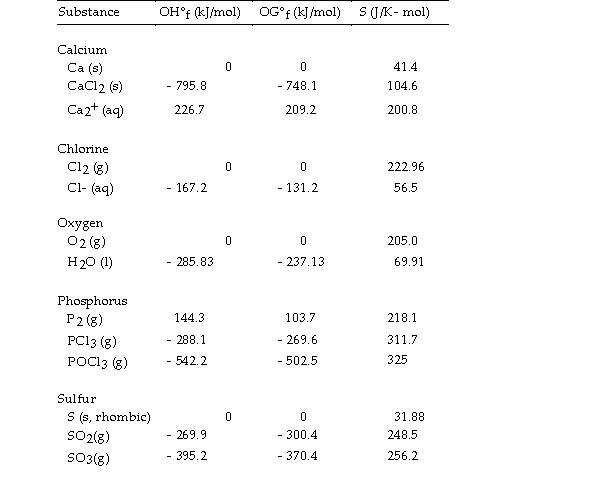
-The value of ΔS° for the oxidation of solid elemental sulfur to gaseous sulfur trioxide,  is J/K.
is J/K.
Definitions:
Marginal Revenue
The additional income obtained from selling one more unit of a product or service.
Deadweight Loss
A loss of economic efficiency that can occur when the equilibrium for a good or a service is not achieved or is unachievable.
Socially Efficient
A situation where resources are allocated in a way that maximizes the total benefit to society.
Monopoly
A market structure characterized by a single seller dominating the market, often leading to higher prices and lower output than in competitive markets.
Q2: The purpose of the salt bridge in
Q13: Of the following solutions, which has the
Q16: The value of ΔS° for the formation
Q21: The dividing line between the troposphere and
Q28: The anode of the alkaline battery is
Q41: The only element with no neutrons is
Q69: The value of ΔS° for the decomposition
Q80: Chromium and chlorine form an ionic compound
Q83: Classify the following compounds as weak acids
Q110: Units of the rate constant of a