A 200-g metal container, insulated on the outside, holds 100 g of water in thermal equilibrium at 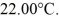 A 21-g ice cube, at the melting point, is dropped into the water, and when thermal equilibrium is reached the temperature is 15.00°C. Assume there is no heat exchange with the surroundings. For water, the specific heat is 4190 J/kg · K and the heat of fusion is 3.34 × 105 J/kg. The specific heat for the metal is closest to
A 21-g ice cube, at the melting point, is dropped into the water, and when thermal equilibrium is reached the temperature is 15.00°C. Assume there is no heat exchange with the surroundings. For water, the specific heat is 4190 J/kg · K and the heat of fusion is 3.34 × 105 J/kg. The specific heat for the metal is closest to
Definitions:
Intraoperative Phase
The period during a surgical procedure, from the time the surgery begins until the patient is transferred to the recovery area.
Sterile Field
An area free of microorganisms and prepared to receive sterile equipment during medical procedures.
Informed Consent
The process of providing comprehensive information about a medical procedure or experiment to a participant, allowing them to decide knowingly whether to participate.
Signed Consent Form
A legal document indicating that a person has been informed about and agrees to a procedure or treatment plan.
Q9: A charge Q = -610 nC is
Q15: Thirteen resistors are connected across points A
Q16: A chunk of ice (T = -20°C)
Q17: An ideal parallel-plate capacitor consists of a
Q17: The number of molecules in one mole
Q29: In the figure, the rotor of a
Q31: A 810-g quantity of ethanol, in the
Q37: 3.0 moles of an ideal gas with
Q55: A 6.00-μF parallel-plate capacitor has charges of
Q88: The figure shows the pV diagram for