An ideal gas in a balloon is kept in thermal equilibrium with its constant-temperature surroundings. How much work is done by the gas if the outside pressure is slowly reduced, allowing the balloon to expand to 6.0 times its original size? The balloon initially has a pressure of 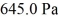 and a volume of
and a volume of 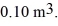 The ideal gas constant is R = 8.314 J/mol ∙ K.
The ideal gas constant is R = 8.314 J/mol ∙ K.
Definitions:
Internet Shopping
The act of purchasing goods or services via the internet through online retailers and marketplaces.
Competitive Market
A market structure characterized by many buyers and sellers, such that no single entity can dictate prices or terms of sale.
Soybean Market
A specific sector of the agricultural market focused on the trading and production of soybeans.
Supply Curve
A graph that shows the relationship between the price of a good and the quantity of the good that suppliers are willing to sell.
Q9: A thin 2.00-m string of mass 50.0
Q10: An 8.0-g bullet is shot into a
Q12: What is the change in entropy of
Q14: Two long parallel wires carry currents of
Q25: A 2.15 kg lightly damped harmonic oscillator
Q41: A weather balloon contains 12.0 m<sup>3</sup> of
Q47: For the circuit shown in the figure,
Q49: The figure shows a pV diagram for
Q53: A solid metal sphere is 15.0 cm
Q96: A bicycle is traveling north at 5.0