Given this equilibrium constant data at 25 C, 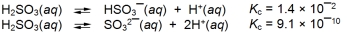 what is the value of Kc at this temperature for the reaction
what is the value of Kc at this temperature for the reaction 
Definitions:
Redeems
The act of repaying or buying back something, such as a company repurchasing its own shares or a bond issuer paying back the principal at maturity.
Discount on Bonds Payable
The difference between the face value of bonds and their selling price when issued at less than their face value.
Journal Entry
A record in the accounting books that represents a transaction and its effect on various accounts, typically involving a debit and credit.
Bond Redemption
The process of repaying the principal amount of a bond at or before its maturity date.
Q2: Potassium is a strong reducing agent.
Q17: Consider the general gas-phase reaction of a
Q20: When a catalyst is added to a
Q35: What is the name of PCl<sub>3</sub>?<br>A) phosphorus
Q36: Select the correct name for this compound.
Q38: The strongest elemental oxidizing agents are found
Q57: What is the name of the acid
Q75: Hydrogen peroxide, H<sub>2</sub>O<sub>2</sub>, is<br>A) used in the
Q84: Consider the following mechanism for the
Q90: The normal boiling point of ether is