What is E°cell for the following reaction, and is it spontaneous or nonspontaneous under standard-state conditions? 2Fe3+(aq) + 2H2O(l) → H2O2(aq) + 2H+(aq) + 2Fe2+(aq)
Given: 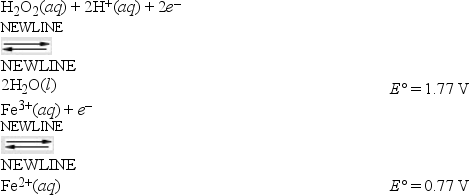
Definitions:
Promissory Estoppel
A principle enforcing a party’s promise to prevent injustice, even if the promise was not formalized into a contract.
Reasonable Reliance
The act of depending on the promise, statement, or action of another on the grounds that it is sensible and justifiable to do so under the circumstances.
Employment Policy Manual
A document provided by employers to employees, outlining workplace rules, policies, and expectations.
Promissory Estoppel
Promissory estoppel is a legal principle that allows a party to recover on a promise made without a formal contract, provided they have relied on that promise to their detriment.
Q8: What is ΔG°<sub>rxn</sub> for the following reaction?
Q31: A polymer made in a polymerization reaction
Q38: The rate constant of the reaction Cl
Q41: Which name could correspond to the following
Q60: What is the ion product expression for
Q87: Sulfuryl dichloride may be formed from the
Q94: What is the equilibrium constant at 25°C
Q103: The temperature at which the following process
Q111: Write the ion product expression for calcium
Q125: Butyric acid is responsible for the odor