What is the standard free-energy change for the following reaction at 25°C? (F = 96,500 C • mol-1) 2Al(s) + 3Sn4+(aq,1 M) → 2Al3+(aq,1 M) + 3Sn2+(aq,1 M) 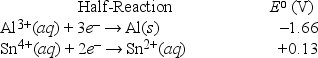
Definitions:
Probability
The measure of the likelihood that an event will occur.
Advertisement
A form of communication aimed at promoting or selling a product, service, or idea.
Antibacterial Soap
A type of cleansing product containing chemical ingredients intended to kill or inhibit the growth of bacteria.
Consumer Survey
A method of collecting data directly from a targeted group of consumers to understand their preferences, behaviors, and opinions on products or services.
Q48: What happens to the solution if sodium
Q60: What is ΔS° for the following reaction?
Q60: Which of the following is not a
Q77: What name is given to the species
Q97: Propanoic acid (CH<sub>3</sub>CH<sub>2</sub>COOH) has a K<sub>a</sub> of
Q99: A patient's thyroid gland is to be
Q103: The temperature at which the following process
Q104: A pure sample of tritium, <sup>3</sup>H, was
Q114: What is E°<sub>cell</sub> for a galvanic cell
Q134: Briefly describe the three main pathways for