For the endothermic reaction A2 (g)  2A(g) , a snapshot of an equilibrium mixture of A(g) and A2 (g) at low temperature may look as follows. (Each circle represents 1.0 mol of A atoms, and the volume of the box is 1.0 L.)
2A(g) , a snapshot of an equilibrium mixture of A(g) and A2 (g) at low temperature may look as follows. (Each circle represents 1.0 mol of A atoms, and the volume of the box is 1.0 L.) 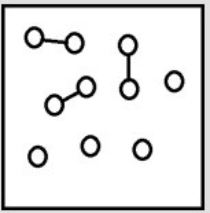 If the temperature is raised, what might the new equilibrium system look like?
If the temperature is raised, what might the new equilibrium system look like?
Definitions:
Population Standard Deviation
Population Standard Deviation measures the dispersion or variability within a set of values for an entire population, indicating how much the individual data points differ from the mean.
Computation Errors
Mistakes or inaccuracies that occur in the process of calculating or processing data.
Sample
A Sample is a subset of individuals or items selected from a larger population for the purpose of statistical analysis.
Standard Deviation
Standard deviation is a measure of the dispersion or variation in a set of values, indicating how much the values deviate from the mean.
Q22: Phosgene, COCl<sub>2</sub>, a poisonous gas, decomposes as
Q29: The strongest intermolecular interactions between ethyl alcohol
Q30: K<sub>w</sub> = 1.0 × 10<sup>-14</sup> under all
Q37: Which is a statement of the second
Q40: Which equation is correct?<br>A) ΔG = ΔG°
Q52: Carbon tetrachloride reacts at high temperatures with
Q54: A 25.0-mL sample of 1.00 M NH<sub>3</sub>
Q80: For the endothermic reaction A<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8482/.jpg"
Q86: _ is the way to identify the
Q124: The following is an Arrhenius plot of