Exhibit 13-12 Consider the aqueous reaction and data below to answer the following question(s) . 2 HgCl2 (aq) + C2O42 - (aq) →2 Cl - (aq) + 2 CO2 (g) + HgCl2 (s) 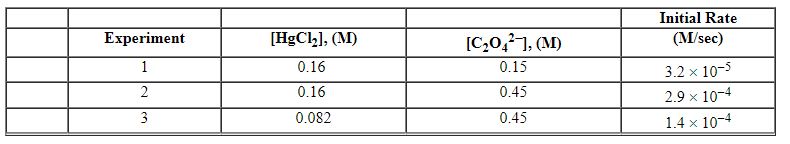
-Refer to Exhibit 13-12. What is the overall order of the rate law?
Definitions:
Equilibrium
A state of balance or stability arising from the equal action of opposing forces.
Self-reinforcement
Administering rewards or punishments to oneself for meeting, exceeding, or falling short of one’s own expectations or standards.
Conscience
An inner feeling or voice viewed as a guide to the rightness or wrongness of one's behavior.
Superego
In Freudian psychoanalysis, the part of the psyche that acts as a moral center, incorporating the values and norms of society and acting as a counterbalance to the id.
Q1: The second period homonuclear diatomic molecule with
Q15: Calculate the molarity of glucose (C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>) in
Q31: Which of the following reactions is product
Q50: Exhibit 15-2 The pH of a solution
Q60: Milk of magnesia is essentially a saturated
Q85: The mole fraction of acetone in a
Q105: A catalyst may be defined as:<br>A) a
Q139: A solution of 1.07 g of NH<sub>4</sub>Cl
Q159: The solubility of CaF<sub>2</sub> in water is
Q164: Some N₂H<sub>4</sub> is placed in a 1.0