Multiple Choice
What is the value of the concentration equilibrium constant, K c (@1000 K) , for the following reaction?
CO (g) + Cl₂ (g) 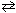 COCl₂ (g) K p = 3.9*10- 2 (@ 1000 K)
COCl₂ (g) K p = 3.9*10- 2 (@ 1000 K)
Understand how to analyze and interpret data presented in graphical formats.
Grasp the concept of slope in graphical analysis and its implication on economic variables.
Recognize the distinction between positive and normative economics.
Identify the characteristics and implications of economic growth.
Definitions:
Related Questions
Q29: Consider the reaction:<br>PCl 3 (g) + Cl₂
Q36: Given the three statements below, pick the
Q38: Consider an aqueous solution that contains the
Q44: What intermolecular force(s) of interaction is(are) present
Q48: The solubility of a substance decreases with
Q88: The total number and type of bonds
Q90: Which of the following aqueous solutions is(are)
Q122: The half-life of the first order radioactive
Q144: Lead(II) chloride has limited solubility in water
Q167: What is the H<sub>3</sub>O<sup>+</sup> concentration in a