The equilibrium constant, K c , for the reaction below is 1.2*1045 at 25 C. CO (g) + 1 / 2 O₂(g) 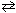 CO₂ (g) What can be stated about the relative equilibrium composition for this mixture?
CO₂ (g) What can be stated about the relative equilibrium composition for this mixture?
Definitions:
Cynically
Relating to the belief that people are motivated purely by self-interest; skeptically.
Open-Mindedly
Approaching ideas, arguments, and experiences without prejudice, being willing to consider different perspectives and changes in opinion.
Self-Contradictory
Describing a statement or situation that contains elements that are logically inconsistent with each other.
Grammatical Construction
The arrangement and relationship of words, phrases, and clauses in sentences, adhering to the rules of a language.
Q7: Consider the following chemical reaction at equilibrium:<br>2
Q41: The half-life of the first order radioactive
Q47: What is the molar solubility of Al(OH)<sub>3</sub>,
Q57: Consider a buffer solution containing a weak
Q59: Exhibit 13-13 Graphical analysis can be used
Q72: Refer to Exhibit 13-2. What is the
Q91: Pepsin is an enzyme present in the
Q110: Refer to Exhibit 13-8. What is the
Q138: How many grams of glucose (C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>) must
Q197: For the reaction C₂ H₆(g) + 7