Consider the following system at equilibrium and its corresponding concentration equilibrium constant:
3 O₂(g) 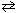 2 O3(g) K c = 1.8*10- 56 at 570 K What is the value of K p for this reaction at 570 K?
2 O3(g) K c = 1.8*10- 56 at 570 K What is the value of K p for this reaction at 570 K?
Definitions:
Leverage
The use of borrowed funds or other financial instruments to increase the potential return of an investment.
Potential Return
The expected gain or loss from an investment, taking into consideration both the risk and the expected rate of return.
Shareholders
Shareholders are individuals or entities that own shares of stock in a corporation, giving them partial ownership and possibly rights to dividends and a say in company matters.
SEC Registration
The process of filing documents required by the Securities and Exchange Commission prior to offering stock for sale to the public.
Q2: Consider the Haber process for preparing ammonia
Q41: The half-life of the first order radioactive
Q56: Which experiment listed below would provide a
Q64: Which molecule listed below would have its
Q80: What intermolecular force(s) is(are) present among molecules
Q93: The gas-phase decomposition of SO<sub>2</sub>Cl<sub>2</sub> is a
Q122: Refer to Exhibit 15-3. What is the
Q149: Which of the following aqueous solutions is(are)
Q167: Given the following two reactions and their
Q196: The solubility of calcium arsenate, Ca<sub>3</sub>(AsO<sub>4</sub>)<sub>2</sub>, in