Exhibit 14-3 Consider the reaction below and its corresponding equilibrium constant at 2000 C to answer the following question(s) :
N₂(g) + O₂(g) 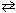 2 NO (g) K c = 4.10*10- 4 (at 2000 C) Complete the table shown below for an experiment where 0.55 moles of N₂is mixed with 0.45 moles of O₂in a 1.00 Liter reaction vessel and the reaction mixture is allowed to come to equilibrium. Concentration N₂O₂ 2 NO Initial 0.55 M 0.45 M Change At equilibrium
2 NO (g) K c = 4.10*10- 4 (at 2000 C) Complete the table shown below for an experiment where 0.55 moles of N₂is mixed with 0.45 moles of O₂in a 1.00 Liter reaction vessel and the reaction mixture is allowed to come to equilibrium. Concentration N₂O₂ 2 NO Initial 0.55 M 0.45 M Change At equilibrium
-Refer to Exhibit 14-3. What equilibrium expression shown below would allow you to solve for x and therefore enable you to solve for the equilibrium concentrations of each of the substances present at equilibrium?
Definitions:
Sobriety
The state of not being under the influence of alcohol or drugs, often used in the context of recovery from addiction.
Group Therapy
A form of psychotherapy that involves one or more therapists working with several individuals at the same time, often beneficial for sharing experiences and learning from others.
Disturbed Sensory Perception
A clinical condition where an individual's perception of sensory input is altered, potentially leading to misinterpretations or hallucinations.
Ineffective Coping
The inability to use strategies or mechanisms that manage or mitigate personal stress, leading to impaired functioning.
Q33: Which of the following would have an
Q41: What is the pH of an aqueous
Q41: What osmotic pressure , p , could
Q43: A reaction will proceed spontaneously at any
Q72: At 25 ° C, 13.0 mg of
Q85: What is the acid ionization constant ,
Q89: In the titration of 0.100 M HCl
Q90: The pepsin-catalyzed hydrolysis of carbobenzene-1-glutamyl-1-tyrosine ethyl ester
Q105: When comparing Bronsted-Lowery acid strengths , which
Q212: The equilibrium constant ( K p )