At 1073 K, the pressure of CO₂ is 0.236 atm when produced by the reaction:
CaCO3(s) 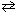 CaO (s) + CO₂ (g) The value of K c for this reaction at 1073 K is (R = 0.0821 L atm/(mol K) ) :
CaO (s) + CO₂ (g) The value of K c for this reaction at 1073 K is (R = 0.0821 L atm/(mol K) ) :
Definitions:
Fiscal Policy
Government strategies to influence a country's economy through public spending and taxation decisions.
Monetary Policy
The process by which the central bank of a country controls the supply of money, often targeting an inflation rate or interest rate to ensure price stability and general trust in the currency.
United States Government Budget
The budget prepared by the federal government, outlining its projected revenues and expenditures for the forthcoming fiscal year.
Fiscal Year
A one-year period used by governments and businesses for accounting and budget purposes, which may not align with the calendar year.
Q20: Which of the following sulfide salts is
Q24: What is the definition for mass percent
Q44: What species are formed in the hydrolysis
Q57: Which colligative property of solutions listed below
Q91: Pepsin is an enzyme present in the
Q109: What species are formed in the hydrolysis
Q122: The half-life of the first order radioactive
Q177: What is the molarity of ions in
Q200: What is the equilibrium constant, K p
Q202: What is the minimum concentration of Br<sup>