Consider the ionization of hydrofluoric acid as follows:
HF + H₂ O 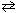 H₃O + + F 1 - What is the effect of adding the common ion, F - (source:
H₃O + + F 1 - What is the effect of adding the common ion, F - (source:
NaF) , to this equilibrium system?
Definitions:
Preschoolers
Children who are in the age range of three to five years, prior to entering formal schooling.
Albert Bandura
A psychologist known for his work on social learning theory and the concept of self-efficacy.
Bobo Doll
An experiment conducted by Albert Bandura demonstrating that children can learn aggressive behaviors through observing and imitating others.
Social Learning
The process of learning behaviors, norms, and values by observing and imitating others within a social context.
Q8: The reaction of chlorine and water vapor
Q11: Phosphorous - 32 has a half-life of
Q11: Consider the reaction:<br>2 NO<sub>2</sub> (g)→N<sub>2</sub>O<sub>4</sub> (g) D
Q14: Reaction of red hot carbon with steam
Q35: Which of the following is associated with
Q42: Consider a voltaic cell that operates from
Q71: Which of the following substances would behave
Q105: What is the molar solubility of PbBr<sub>2</sub>
Q140: When the reversible reaction, N₂+ O₂ <img
Q150: At equilibrium, the endothermic reaction N₂(g) +