Multiple Choice
What volume of 0.200 M H₂ SO₄is required to neutralize 50.0 mL of 0.100 M NaOH?
Assume the chemical equation is:
H₂ SO₄(aq) + 2 NaOH (aq) Na2 SO₄(aq) + 2 H₂ O ( 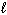 )
)
Definitions:
Related Questions
Q21: In the titration of 25.0 mL of
Q42: Consider the following equilibrium reaction:<br>NH₃+ H₂ O
Q55: What is the conjugate acid of the
Q74: Given:<br>2 U<sup>3+</sup> + Cd<sup>2+</sup>→Cd (s) + 2
Q88: At a minimum, what experimental data is
Q97: A sample of hydrochloric acid (approximately 0.10
Q111: The value of K p is 2.72
Q117: For the reaction Fe (s) + 4
Q118: The equilibrium constant, K c , for
Q135: After completing the following unfinished Base Ionization