Exhibit 16-3 Consider the Titration curve below for the titration of a weak acid ,CH ₃COOH , with a strong base , NaOH, to answer the following question(s) . CH ₃COOH + NaOH CH ₃COONa + H₂ O 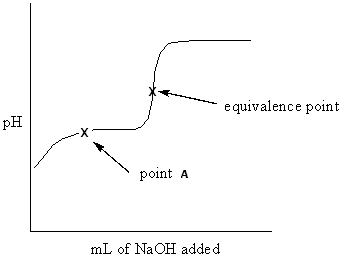
Refer to Exhibit 16-3. What approach listed below would be used to determine the pH at point A of the solution?
Definitions:
River Terrace
A flat, bench-like structure alongside a river, formed by the river cutting deeply into the land then retreating, leaving a raised platform.
Well-developed Soil
Soil that has undergone extensive formation processes, resulting in distinct layers and rich nutrient content.
Lava Flow
The movement of lava as it pours out and spreads over the land following a volcanic eruption.
Angular Unconformity
An unconformity (ancient erosion surface) in which the older, underlying strata dip more steeply or at a different angle than the younger, overlying strata.
Q7: A special type of bonding in the
Q8: A sample of parchment produces 10.3 disintegrations
Q14: Reaction of red hot carbon with steam
Q15: Which of the following ligands might form
Q45: The atomic mass of beryllium is 9.012.
Q53: The equilibrium constant for the reaction PCl<sub>5</sub>
Q70: What is the molar solubility of Ag<sub>2</sub>SO<sub>4</sub>
Q106: What is the correct IUPAC name for
Q192: Which system at equilibrium shifts right ?<br>I.
Q210: What would be the equilibrium constant expression