If we know the amount of light that is absorbed in a solution we can calculate the concentration of the substance dissolved in water using a spectrophotometer. For a certain substance the concentration (in moles/liter) is given by the formula  where
where 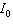 is the intensity of the incident light and
is the intensity of the incident light and 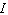 is the intensity of light that emerges. What is the concentration of the substance if the intensity
is the intensity of light that emerges. What is the concentration of the substance if the intensity 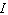 is 45% of
is 45% of 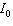 ?
?
Definitions:
Environmental Regulations
Rules and standards implemented by governments to control pollution and protect the environment.
Annual Statement
A comprehensive report detailing a company’s financial performance over the previous year, including income, expenses, and profit or loss.
Drinking Water
Water that is safe and clean for human consumption, free from contaminants and pollutants.
Toxic Chemicals
Substances that can cause harm to humans, animals, plants, or the environment.
Q16: Determine whether the equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8673/.jpg" alt="Determine
Q25: For the given input-output table complete the
Q31: Determine the equation of the line that
Q63: Solve the logarithmic equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8673/.jpg" alt="Solve
Q132: The solution to the equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8673/.jpg"
Q194: The expression <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8673/.jpg" alt="The expression
Q194: If we know the amount of light
Q234: A system of equations can only be
Q256: If solving a system of equations using
Q258: Divide the expressions. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBX8673/.jpg" alt="Divide the