Carbon tetrachloride reacts at high temperatures with oxygen to produce two toxic gases, phosgene and chlorine. CCl4(g) + 1/2O2(g) 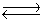 COCl2(g) + Cl2(g) , Kc = 4.4 * 109 at 1,000 K
COCl2(g) + Cl2(g) , Kc = 4.4 * 109 at 1,000 K
Calculate Kc for the reaction 2CCl4(g) + O2(g) 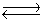 2COCl2(g) + 2Cl2(g) .
2COCl2(g) + 2Cl2(g) .
Definitions:
Collective Responsibility
The concept that individuals in a group share responsibility for actions taken by the group as a whole, including the outcomes of those actions.
Shared Leisure Interests
Shared leisure interests refer to common activities or hobbies that people enjoy during their free time, forming a basis for social connections and community.
Robert Bellah
An American sociologist known for his work on the role of religion in modern society, particularly through the concept of civil religion.
Leisure
Free time when an individual is not working or fulfilling obligations and can choose activities for relaxation, enjoyment, or other personal interests.
Q10: The molar enthalpy of vaporization of carbon
Q21: Which one of these statements about strong
Q34: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q53: Using the thermodynamic data provided below, calculate
Q69: Identify the conjugate acid of CO<sub>3</sub><sup>2-</sup>
Q78: Identify the dominant (strongest)type of intermolecular force
Q82: K<sub>c</sub> for the reaction CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img
Q87: Which one of the following statements is
Q89: Arrange the following substances in the
Q158: Will a 0.1 M solution of NaH<sub>2</sub>PO<sub>4</sub>(aq)be