Consider the following standard reduction potentials in acid solution: 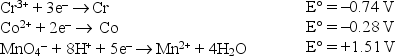 The weakest reducing agent listed above is
The weakest reducing agent listed above is
Definitions:
Q9: Balance the equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3244/.jpg" alt="Balance the
Q31: Which one of these choices is the
Q43: Consider the weak bases below and their
Q50: Consider the following equilibrium,<br>4NH<sub>3</sub>(g)+ 3O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3244/.jpg"
Q52: Write the chemical formula for sulfuric acid.
Q85: A solution was prepared such that the
Q94: For the reaction SbCl<sub>5</sub>(g) <span
Q102: Radioactive nitrogen-13 has a half-life of 10
Q102: Consider the following standard reduction potentials in
Q145: Will a 0.1 M solution of Na<sub>2</sub>HPO<sub>4</sub>(aq)be