The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 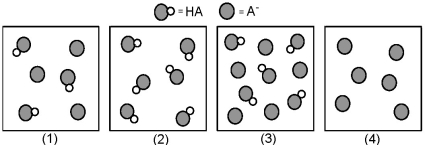
-Which solution has the highest pH?
Definitions:
Auxiliary Consumption
Secondary or supplemental use of products or services that support or enhance the primary consumption activities.
Production
The process of creating, manufacturing, or otherwise bringing goods or content into existence.
Emotional Engagement
The emotional connection and response that a consumer has with a brand, which can influence loyalty and purchasing behavior.
Self-Identification
The process by which an individual defines and understands themselves, often in relation to categories such as gender, race, or religion.
Q32: Using the following standard reduction potentials<br>Fe<sup>3+</sup>(aq)+ e<sup>-</sup>
Q46: What is the strongest acid among the
Q54: Which solution has the lowest pH?<br>A)(1)<br>B)(2)<br>C)(3)<br>D)(4)
Q75: Which solution has the largest percent dissociation
Q89: K<sub>c</sub> is 1.67 × 10<sup>20</sup> at 25°C
Q125: Which statement is true concerning the standard
Q125: The following picture represents the equilibrium state
Q133: What is the equilibrium constant expression for
Q138: What is the pH of a solution
Q185: What is the percent dissociation of glycine