The following pictures represent solutions that contain a weak acid HA (pKa = 5.0) and its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 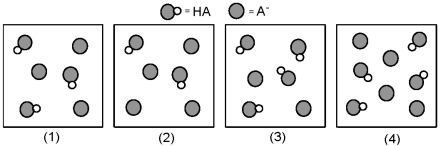
-Which of these solutions are buffers?
Definitions:
Q4: Which solution has the greatest buffer capacity?<br>A)(1)<br>B)(2)<br>C)(3)<br>D)(4)
Q49: The solubility of 1:1 salts is measured
Q63: Which point a-d represents the first equivalence
Q71: Calculate the hydroxide ion concentration in an
Q78: Which nonequilibrium mixtures will react in the
Q83: What is the hydronium ion concentration and
Q94: For initial state 1 what is the
Q97: A reaction reaches dynamic equilibrium at a
Q139: Which of the following is zero at
Q224: At 25°C,the pH of a vinegar solution