The following pictures represent solutions of CaCO3,which may also contain ions other than Ca2+ and CO32- which are not shown.Gray spheres represent Ca2+ ions and unshaded spheres represent CO32- ions. 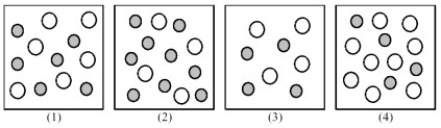
-If solution (1) is a saturated solution of CaCO3,which of solutions (1) -(4) represents the solution after a small amount of Ca(NO3) 2 is added and equilibrium is restored?
Definitions:
Routine Business Requests
Standard or common requests made in the course of business operations, such as for information or action.
Requesting a Reference
The act of asking someone, typically a former employer or professional contact, to provide a positive account of your abilities or character to a potential employer.
Routine Messages
Regular, standardized communications used for everyday business operations.
Positive Messages
Communications that convey good news, approval, or optimistic information.
Q7: The following picture represents the equilibrium state
Q15: Bromothymol blue indicator changes color from yellow
Q22: If the concentrations of Ag<sup>+</sup>(aq)and Cu<sup>2+</sup>(aq)are varied
Q25: Which of the above reaction mixtures has
Q26: The enthalpy for the following reaction is
Q53: What is the pH of a solution
Q66: If solution (1)is a saturated solution of
Q98: Standard molar entropies,S°,in J/Kmol,are given below each
Q173: The initial concentrations of Ag<sup>+</sup>(aq)and Cu<sup>2+</sup>(aq)are both
Q195: CO<sub>2</sub> reacts with H<sub>2</sub>O to form HCO<sub>3</sub><sup>-</sup>