Shown below is a galvanic cell with anode compartment b containing anode a and cathode compartment d containing cathode c.Electrons flow through wire f,ions flow through salt bridge e,and the cell potential is read using voltmeter g.
This galvanic cell uses the reaction: Cu(s) + 2 Ag+(aq) 2 Ag(s) + Cu2+(aq) . 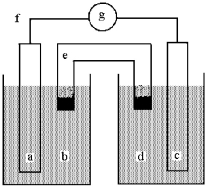
-The initial concentrations of Ag+(aq) and Cu2+(aq) are both 1.0 M.What will happen to the cell voltage if 1.0 M Cu(NO3) 2 is added to the compartment containing the 1.0 M Cu2+(aq) ? The cell voltage will
Definitions:
Relevant Education
Educational experiences or content that directly pertains to a specific subject, skill, or field of interest, making it significant or applicable to the task or situation at hand.
Position
A place where someone or something is located or has been put, or a particular stance taken on an issue.
Expert Testimony
Evidence provided in court by a qualified professional on subjects requiring specialized knowledge.
Objective
Something that is not influenced by personal feelings or opinions and is based on facts; often a goal that is aimed to be achieved.
Q6: Precipitation of an ionic compound will occur
Q8: In which of the following solutions would
Q28: The entropy change associated with the expansion
Q39: Elements in period 2 of the periodic
Q50: Addition of 0.0125 mol KOH to 150
Q67: An electron in an oxygen p orbital
Q99: Which solution has the highest pH?<br>A)(1)<br>B)(2)<br>C)(3)<br>D)(4)
Q107: If solution (1)is a saturated solution of
Q146: Which oxide has the highest melting point?<br>A)A<br>B)B<br>C)C<br>D)D
Q148: What is the most metallic element of