At 20 °C,an aqueous solution that is 24.0% by mass in ammonium chloride has a density of 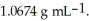 What is the molarity of ammonium chloride in the solution?
What is the molarity of ammonium chloride in the solution?
The formula weight of 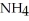 Cl is
Cl is 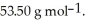
Definitions:
Goods Exports
The act of sending domestically produced goods to another country for sale or trade.
Current Account
A country's transactions with the rest of the world, including goods, services, income, and current transfers.
Net Inflow
Net inflow refers to the total incoming resources or capital minus the outgoing resources or capital in a particular time period.
Q9: Based on the figure above,the boiling point
Q30: An equilibrium mixture of CO,O<sub>2</sub>,and CO<sub>2</sub> at
Q47: Surface tension is thought to be due
Q69: Gold crystallizes in a face-centred cubic structure.What
Q70: The solubility product constant of silver bromide
Q73: Unhybridized p orbitals must be present for
Q79: For a reaction,the reaction quotient,Q<sub>c</sub> > K<sub>c</sub>
Q86: A 250.0 ml sample of gaseous hydrogen
Q94: The enthalpy change for converting 1.00 mol
Q111: pOH = 3.14 is equivalent to:<br>A)pH =