An aqueous solution is prepared by dissolving 0.23 mol of hydrazoic acid and 0.27 mol of sodium azide in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of NaOH to this buffer solution causes the pH to increase slightly.The pH does not increase drastically because the NaOH reacts with the ________ present in the buffer solution.The 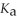
Of hydrazoic acid is 1.9 × 10-5.
Definitions:
Illegal Contract
An agreement that is against the law or public policy and therefore unenforceable in a court of law.
Performance Rendered
The execution or completion of a task or duty as agreed in a contract or agreement.
Unconscionable Contract
An agreement that is so unfair or oppressive to one party that it is deemed unjust by the legal system.
Grossly Unfair
A situation or action that is extremely unjust or disproportionate, often violating ethical or legal standards.
Q2: HNO<sub>3</sub> is a strong acid.
Q12: For the second order reaction A →
Q35: Calculate the freezing point of a solution
Q39: For the second order reaction A →
Q40: For the production of NO<sub>2</sub>,K<sub>c</sub> = [NO<sub>2</sub>]<sup>2</sup>/[NO]<sup>2</sup>[O<sub>2</sub>].At
Q73: What is the balanced equation for the
Q75: Which of the following aqueous solutions has
Q105: Choose the INCORRECT statement.<br>A)Elemental silicon is produced
Q111: What is the pH of an aqueous
Q112: The term pH = -ln [H<sup>+</sup>].