Calculate the pH of an aqueous solution that is 0.295 mol L-1 in sodium formate (HCOONa) and 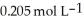 In formic acid (HCOOH) .The
In formic acid (HCOOH) .The 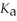 Of formic acid is 1.77 ×
Of formic acid is 1.77 × 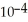 .
.
Definitions:
Concentration
Refers to the amount of a substance per defined space or unit volume.
Hypertonic
Describes a solution that has a higher concentration of solutes compared to another solution, causing cells to lose water.
Hypotonic
Describing a solution with a lower solute concentration compared to another solution, leading to water influx if two solutions are separated by a semipermeable membrane.
Isotonic
Describing a solution that has the same osmotic pressure as another solution, leading to no net movement of water across a cell membrane.
Q2: At a given temperature the vapour pressures
Q15: Determine the [F<sup>-</sup>] of the following aqueous
Q61: A 0.0925 g sample of a monoprotic
Q63: In a qualitative cation analysis,the unknown ion
Q68: Calculate the percent ionization of nitrous acid
Q73: Saturated aqueous solutions of sodium sulfate,ammonium carbonate,and
Q78: Consider an aqueous solution which is 0.020
Q94: Determine the pH of the following aqueous
Q99: What is the composition of the precipitate
Q103: If a catalyst is added to a