Kp for the reaction 4CuO(s) 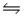 2Cu2O(s) + O2(g) is 0.49 at 1024 °C.Calculate Kc at this temperature
2Cu2O(s) + O2(g) is 0.49 at 1024 °C.Calculate Kc at this temperature
Definitions:
Cost Curve
A graphical representation that shows the cost of producing different quantities of a good or service.
Minimizing Losses
Minimizing Losses involves strategies and actions a business or individual might employ to reduce financial setbacks or decrease the severity of financial downturns.
Total Loss
The complete depletion of value of an asset or the sum of expenses surpassing all revenues, resulting in no net gain.
Most Efficiently
The method or process that achieves the best possible outcome with the least waste of resources.
Q14: For the reaction CuS(s)+ H<sub>2</sub>(g) <img
Q20: What is the pH of a solution
Q41: Identify the conjugate base of HCO<sub>3</sub><sup>-</sup><br>A)H<sub>2</sub>CO<sub>3</sub><br>B)CO<sub>3</sub><sup>2-</sup><br>C)OH<sup>-</sup><br>D)CO<sub>2</sub><br>E)CO
Q45: Calculate the free energy of formation
Q52: The solubility of a salt increases as
Q63: The electrons in the delocalized molecular orbitals
Q74: Equilibrium constants are known for the following
Q111: Calculate the pH of 2.6 × 10<sup>-2</sup>
Q128: Which of the following correctly lists species
Q169: Given the following K<sub>b</sub> values, which cation