At 250ºC, the equilibrium constant Kp for the reaction PCl5(g) 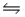 PCl3(g) + Cl2(g) is 1.80.Sufficient PCl5 is put into a reaction vessel to give an initial pressure of 2.74 atm at 250ºC.Calculate the pressure of PCl5 after the system has reached equilibrium.
PCl3(g) + Cl2(g) is 1.80.Sufficient PCl5 is put into a reaction vessel to give an initial pressure of 2.74 atm at 250ºC.Calculate the pressure of PCl5 after the system has reached equilibrium.
Definitions:
Social Settings
The environments or contexts in which social interaction occurs, including cultural, institutional, and interpersonal spaces.
Delay of Gratification
The ability to resist an immediate reward in preference for a later, often greater, reward, reflecting self-control and forward planning.
Academic and Social Competence
The ability to achieve in academic environments and to successfully interact socially with others.
General Coping Ability
The overall capability of an individual to manage stress, adversity, or change effectively.
Q9: Which response has both answers correct? Will
Q12: Ozone (O<sub>3</sub>)in the atmosphere can reaction
Q38: Nitrous oxide (N<sub>2</sub>O)decomposes at 600°C according
Q47: For the following reaction at equilibrium
Q53: Which response includes all of the
Q67: For the reaction H<sub>2</sub>(g)+ S(s) <span
Q74: A saturated sodium carbonate solution at 100°C
Q80: A saturated sodium carbonate solution at 0°C
Q115: Which one of the following substances is
Q120: The intermolecular forces present in C<sub>6</sub>H<sub>6</sub> include