For the equilibrium reaction 2SO2(g) + O2(g) 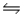
2SO3(g) , Hºrxn = -198 kJ/mol.Which one of these factors would cause the equilibrium constant to increase?
Definitions:
Cnidarians
A phylum of animals that are more complex than sponges, characterized by their radial symmetry, a central gastrovascular cavity, and the presence of cnidocytes; includes jellyfish, corals, and sea anemones.
Spiracles
External respiratory openings found in some animals, such as insects and some amphibians, that allow air to enter and exit the trachea.
Tracheae
Tubes in the respiratory system that conduct air to and from the lungs.
Book Lungs
Respiratory organs found in certain arachnids, such as spiders and scorpions, consisting of stacked leaf-like structures used for gas exchange.
Q6: The equilibrium constant for the reaction C<sub>7</sub>H<sub>15</sub>COOH(aq)+
Q11: SrF<sub>2</sub> crystallizes such that the Sr<sup>2+</sup> ions
Q25: What mass of sodium fluoride must be
Q42: What is the molar mass of toluene
Q45: Potassium crystallizes in a body-centered cubic lattice.How
Q62: Find the temperature at which water boils
Q76: The intermolecular forces present in CH<sub>3</sub>NH<sub>2</sub> include
Q115: Which solution will have the lowest pH?<br>A)0.25
Q129: Platinum has a face-centered cubic crystal structure
Q167: The pH of coffee is approximately 5.0.How