Predict the direction in which the equilibrium will lie for the reaction H3PO4 + NO3-
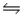
H2PO4- + HNO3 Ka(H3PO4) = 7.5 × 10-3
Definitions:
Glass-Steagall Act
A law enacted in 1933 to separate commercial banking from investment banking, intended to reduce conflicts of interest and prevent bank failures.
Q8: The solubility product for calcium phosphate is
Q48: Equilibrium is established for the reaction 2X(s)+
Q49: NaCl is added slowly to a solution
Q53: For the following exothermic reaction, the
Q72: Complete and balance the following redox
Q74: Equilibrium constants are known for the following
Q86: Determine the equilibrium constant for the following
Q101: Which would have the stronger intermolecular forces
Q116: Which of the following is consistent
Q132: Calculate the pOH for a solution with