The equilibrium constant for the reaction C7H15COOH(aq) + HCOO-(aq) 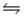
C7H15COO-(aq) + HCOOH(aq)
Is 7.23 × 10-2 at 25°C.If Ka for formic acid (HCOOH) is 1.77 × 10-4, what is the acid dissociation constant for C7H15COOH?
Definitions:
Menstruate
The process in which blood and other materials from the lining of the uterus are discharged externally, occurring in most females as part of the monthly menstrual cycle.
Biological Reductionism
A perspective in psychology and other sciences that seeks to explain complex phenomena in terms of simpler biological processes.
Evolutionary Theory
A theoretical framework that explains how biological traits and behaviors evolve over generations through natural selection, genetic drift, mutation, and gene flow.
Sibling Conflict
The disagreements, competition, and fights that occur between brothers and sisters, often part of normal sibling dynamics.
Q16: For the reaction CuS(s)+ H<sub>2</sub>(g) <img
Q36: If the reaction 2H<sub>2</sub>S(g)<sub> </sub> <sub> </sub>
Q46: An organic compound was prepared and purified
Q55: The following mechanism has been suggested
Q58: A 9.50 % by mass solution of
Q66: What is the pH of a solution
Q82: You have 500.0 mL of a buffer
Q82: How many grams of copper are deposited
Q118: Under which of the following conditions
Q128: Which one of the following reactions