For the reaction 2 SO2(g) + O2(g) 2 SO3(g) , if initially P(SO2) = 1.2 atm, P(O2) = 1.8 atm, and P(SO3) = 2.1 atm, calculate G for this reaction at 25°C.The following data is valid at 25°C: 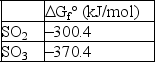
Definitions:
Protein
Protein is a macromolecule composed of amino acids linked by peptide bonds, essential for the structure, function, and regulation of the body's cells, tissues, and organs.
Hemoglobin
A component of red blood cells, hemoglobin serves the crucial function of delivering oxygen from the lungs to tissues across the body and returning carbon dioxide from the tissues to the lungs.
Collagen
The main structural protein found in skin and other connective tissues, providing strength and elasticity.
Enzymes
Biological catalysts that speed up chemical reactions in the body without being consumed by the reactions.
Q2: Calculate the percent ionization of cyanic acid,
Q3: How much energy (kJ)is released if
Q6: The equilibrium constant for the reaction C<sub>7</sub>H<sub>15</sub>COOH(aq)+
Q34: A chemical formula of the carbide ion
Q39: For the reaction 2NO(g)+ O<sub>2</sub>(g) <span
Q45: Which one of the following would slow
Q65: Concerning the following reaction at equilibrium: 3Fe(s)+
Q74: Nitric acid is formed by the
Q103: Arrange the acids HOBr, HBrO<sub>3</sub>, and HBrO<sub>2</sub>
Q107: Use the table of data shown