Consider the reaction CO(g)+ 2H2(g) 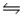
CH3OH(l)at 25°C.
G°f (CO)= -137.3 kJ/mol
G°f (CH3OH)= -166.3 kJ/mol
H°f (CO)= -110.5 kJ/mol
H°f (CH3OH)= -238.7 kJ/mol
S°(CO)= 197.9 J/K·mol
S°(CH3OH)= 126.8 J/K·mol
Calculate value of the equilibrium constant (Kp)for this reaction at 25°C.
Definitions:
Paving Cost
Expenses related to the laying of a pavement, including materials and labor.
Land Improvements
Enhancements made to a parcel of land to increase its value, such as landscaping, fencing, and infrastructure development.
Revenue Expenditure
Expenditures that are immediately charged against revenues as an expense of the period in which they are incurred, often related to the costs of day-to-day operations.
Open Pickup Truck
A light-duty truck with an open cargo area in the rear, often used for transporting goods and materials.
Q8: The element oxygen was prepared by
Q17: In which one of the following solutions
Q32: Which of the following diagrams represents an
Q39: Concerning the following reaction at equilibrium: 3Fe(s)+
Q50: Consider the reaction CO(g)+ 2H<sub>2</sub>(g) <img
Q52: At 250ºC, the equilibrium constant K<sub>p</sub> for
Q70: Consider the reaction N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q83: When the reaction 2H<sub>2</sub>S(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg" alt="When
Q105: How many coulombs (C)of electrical charge must
Q112: How many grams of nickel would be