At 700 K, the equilibrium constant for the reaction CO(g)+ H2O(g) 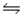
CO2(g)+ H2(g)is 5.10.What is G° for this reaction at this temperature?
Definitions:
Cost Formula
A mathematical equation or method used to calculate the total cost of production or service, considering both variable and fixed expenses.
Activity Levels
The degree of operations or production volume within a specific period, affecting costs and decision-making in businesses.
Variable Cost
Costs that fluctuate in direct proportion to production or sales figures, including items like direct labor and raw materials.
Mixed Costs
Expenses comprising both fixed and variable components, changing in total with the level of activity but not proportionately.
Q6: Consider this reaction at equilibrium: 2SO<sub>2</sub>(g)+
Q22: What is carborundum, and how is it
Q23: Hydrogen iodide decomposes according to the equation:<br>2HI(g)
Q35: The total number of electrons in the
Q42: A current of 0.80 A was applied
Q45: The concentration of Mg<sup>2+</sup> in seawater is
Q76: The equilibrium constants for the chemical
Q83: A 5.5 L sample of a 0.25
Q111: Calculate the pH of a solution that
Q178: Calculate the pH of a 1.6 M