The brown gas NO2 and the colorless gas N2O4 exist in equilibrium, 2NO2 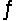 N2O4. In an experiment, 0.625 mole of N2O4 was introduced into a 5.00 L vessel and was allowed to decompose until equilibrium was reached. The concentration of N2O4 at equilibrium was 0.0750 M. Calculate Kc for the reaction.
N2O4. In an experiment, 0.625 mole of N2O4 was introduced into a 5.00 L vessel and was allowed to decompose until equilibrium was reached. The concentration of N2O4 at equilibrium was 0.0750 M. Calculate Kc for the reaction.
Definitions:
Q16: K<sub>c</sub> for the reaction CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img
Q17: Which of the following gives the molarity
Q17: When the following reaction is at equilibrium,
Q69: Calculate the molality of 6.0 M H<sub>2</sub>SO<sub>4</sub>
Q76: Consider the following equilibrium,<br>4NH<sub>3</sub>(g)+ 3O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q77: When 12.1 g of the sugar sucrose
Q81: Indicate the type of hybrid orbitals used
Q91: Bromothymol blue is a common acid-base indicator.
Q97: Rain collected on a remote island in
Q131: Choose the response that lists the member